Synthesis of two reagents for an efficient separation of chemical intermediates

Domaines
Alimentation
Santé et Bien-être
Technologie Procédés relatifs à la chimie verte
Challenges
The challenges for this kind of synthesis are:
- The difficulty in effectively separating racemic enantiomers under mild conditions
- The limited availability of chiral reagents
- The need to optimize yields without compromising enantiomeric purity
- The complexity in managing multi-step processes with broad substrate compatibility
Innovative solution
Unique chiral reagent enabling both separation and deoxygenation of racemic alcohols in two steps.
Applications
-
Synthesis of complex bioactive molecules,
-
pharmaceuticals,
-
fine chemistry,
-
multi-step synthesis,
-
agrochemicals
COMPETITIVE ADVANTAGES
- Easy enantiomer separation via silica gel purification
- Dual functionality: separation and deoxygenation
- Radical reaction tolerant to a wide range of substrates
- Time and step reduction in asymmetric synthesis
- Direct impact on the production of essential enantiopure molecules
How it works
The synthesis process of the separation agent, subject of the invention, allows for the production of two different reagents (3 and 9), which can be used depending on the alcohols to be separated.
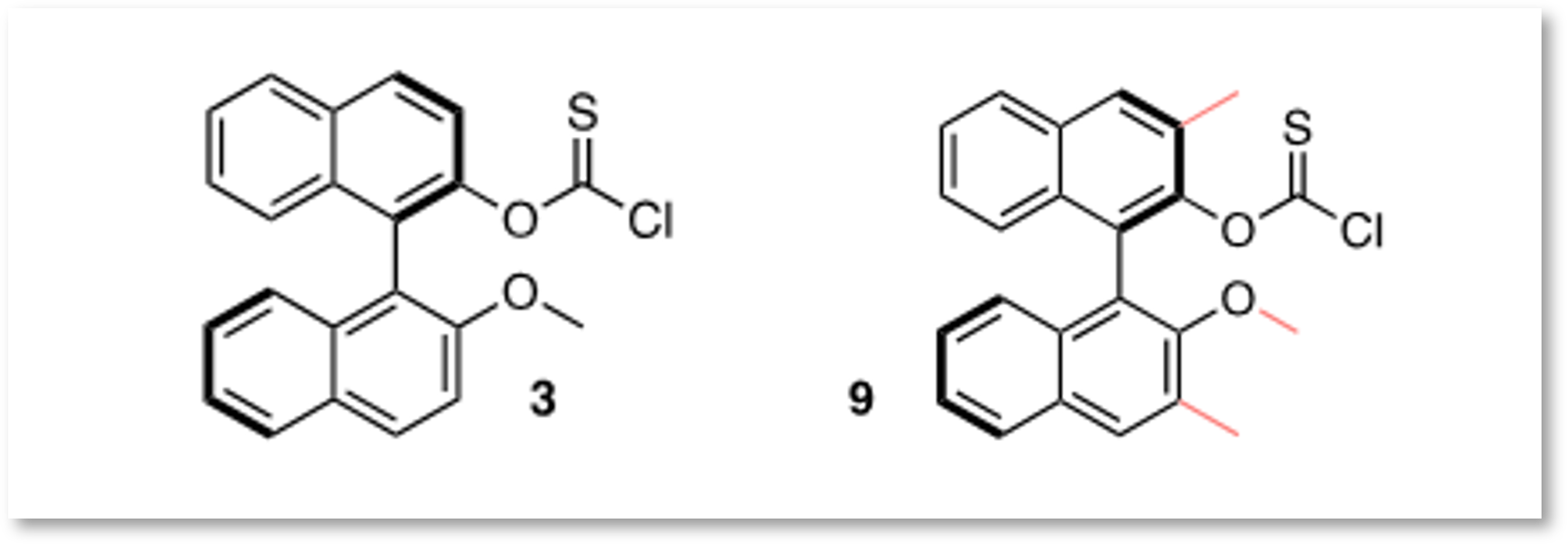
In the example illustrated below, the synthesized reagent 9 is used on the alcohol 13: (+)-myrioneurinol. The two resulting diastereomers can then be separated. Finally, the Barton-McCombie reaction of each compound can be performed, with an excellent yield exceeding 90%.

DEVELOPMENT STATUS - TRL 3
Inventeurs
Philippe PEIXOTO : ISM (university of Bordeaux, CNRS, Bordeaux INP)
Propriété intellectuelle
Patent filed in 2024
PARTNERSHIPS
We are looking for a company interested in engaging in the co-development of this project.
Contact
Carlos LARRAYA
%63%2e%6c%61%72%72%61%79%61%40%61%73%74%2d%69%6e%6e%6f%76%61%74%69%6f%6e%73%2e%63%6f%6d
+33 (0)6 18 40 63 51